
BIMZELX is indicated for the treatment of: moderate to severe plaque PsO in adults who are candidates for systemic therapy; active PsA, alone or in combination with methotrexate, in adults who have had an inadequate response, or who have been intolerant, to one or more DMARDs; active nr-axSpA with objective signs of inflammation as indicated by elevated CRP and/or MRI, in adults who have responded inadequately, or are intolerant, to NSAIDs; active AS in adults who have responded inadequately or are intolerant to conventional therapy; and active moderate to severe HS (acne inversa) in adults with an inadequate response to conventional systemic HS therapy.
Overview
NO COMPROMISE, JUST CLEARANCE
BIMZELX®▼ (bimekizumab) offers the opportunity for complete, fast, and lasting skin clearance and proven PsA efficacy1–7
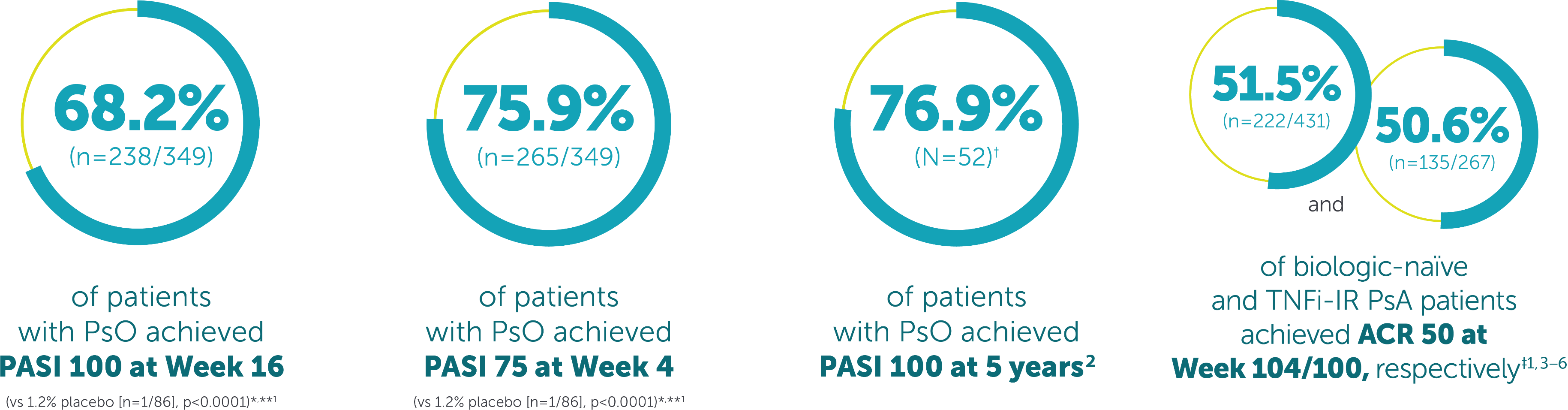
BIMZELX was well tolerated, the most frequently reported adverse reactions were: upper respiratory tract infections and oral candidiasis. Other common reported adverse reactions include tinea infections, ear infections, herpes simplex infections, oropharyngeal candidiasis, gastroenteritis, folliculitis, headache, rash, dermatitis, eczema, acne, injection site reactions, fatigue, and vulvovaginal mycotic infection (including vulvovaginal candidiasis).4
These data are from different clinical trials and cannot be directly compared.
*Co-primary endpoints PASI 90 and IGA 0/1 at Week 16 were met.**Secondary endpoints. †N= mNRI, missing data were imputed with mNRI (patients with missing data following treatment discontinuation due to lack of efficacy or a TRAE were counted as non-responders; multiple imputation methodology was used for other missing data). ‡43.9% (n=189/431), and 43.4% (n=116/267) of biologic-naïve and TNFi-IR PsA patients achieved the primary endpoint of ACR 50 at Week 16 in BE OPTIMAL and BE COMPLETE, respectively (vs 10.0% [n=28/281] and 6.8% [n=9/133] placebo, p<0.0001); 54.5% (n=235/431) and 51.7% (n=138/267) maintained it at Week 52 (NRI).4–6
ACR 50, ≥50% response in the American College of Rheumatology criteria; AS, ankylosing spondylitis; CRP, C-reactive protein; DMARD, disease-modifying antirheumatic drug; HS, hidradenitis suppurativa; IGA, Investigator’s Global Assessment; (m)NRI, (modified) non-responder imputation; MRI, magnetic resonance imaging; nr-axSpA, non-radiographic axial spondyloarthritis; NSAID, non-steroidal anti-inflammatory drug; PASI 75/90/100, ≥75/90/100% improvement from baseline in Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsD, psoriatic disease; PsO, psoriasis; TNFi-IR, tumour necrosis factor-α inhibitor – inadequate responder; TRAE, treatment-related adverse event.
References: 1. Gordon KB, et al. Lancet. 2021;397(10273):475–486. 2. Blauvelt. 2025. AAD Presentation 62275. 3. Mease PJ, et al. Rheumatol Ther. 2024;11(5):1363–1382. 4. BIMZELX SmPC. 5. Ritchlin CT, et al. Ann Rheum Dis. 2023;82(11):1404–1414. 6. Coates LC, et al. RMD Open. 2024;10(1):e003855. 7. Strober B, et al. AAD 2024;oral presentation.
Window of Opportunity
The ‘window of opportunity’ is where timely action offers the chance to reshape the trajectory of psoriatic disease
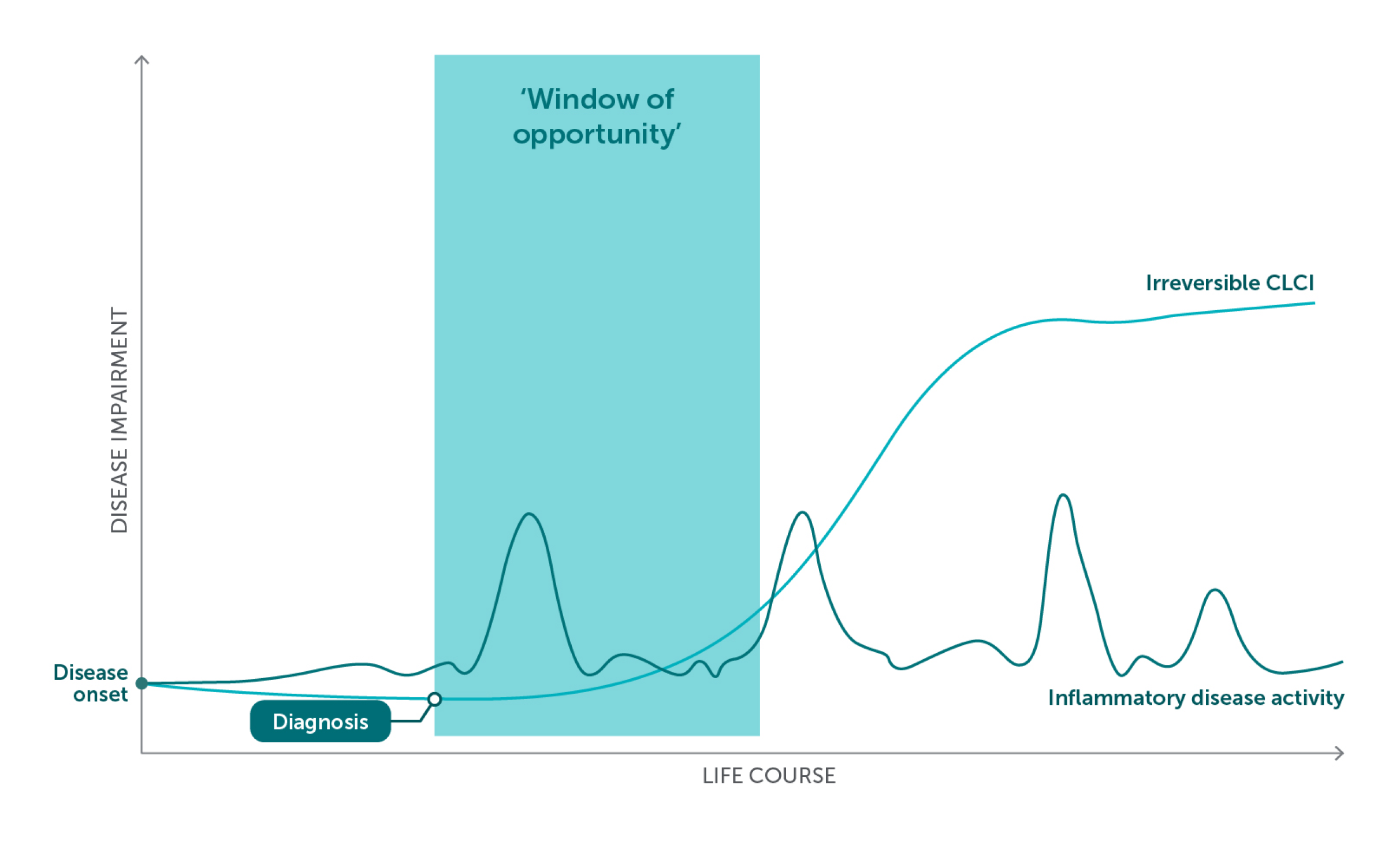
Figure adapted from References 4–7.
In PSO, early intervention with biologics may modulate gene expression and the immune cells infiltrating the skin.4
Biologics act directly on the underlying inflammation and may help to:4
- Slow disease progression, including to psoriatic arthritis
- Reduce PSO-related comorbidities
- Improve patients’ QoL and reduce CLCI4
A targeted and proactive approach may help to avoid disease progression.1–3
CLCI, cumulative life course impairment; PSO, psoriasis; QoL, quality of life.
References: 1. Gisondi P, et al. Dermatol Ther (Heidelb). 2021;11:235–52. 2. Zabotti A, et al. Ann Rheum Dis. 2023;82:1162–70. 3. Hackett S, et al. Ther Adv Musculoskelet Dis. 2022;14:1759720x221086710. 4. Arancio LMH, et al. Clin Exp Dermatol. 2024;49:1525–31. 5. Carter L, et al. J Invest Dermatol. 2022;142:944–50. 6. Felix PAO, et al. Front Med (Lausanne). 2022;9:1027347. 7. Martorell A, et al. Actas Dermosifiliogr. 2016;107:32–42. 8. Angsana J, et al. 2023. ISID. Poster 587. 9. Schäkel K, et al. 2024. AAD. Poster 50236.
MOA
BIMZELX offers the chance to act within the ‘window of opportunity’ through its differentiated MoA and systemic inflammatory control1–4
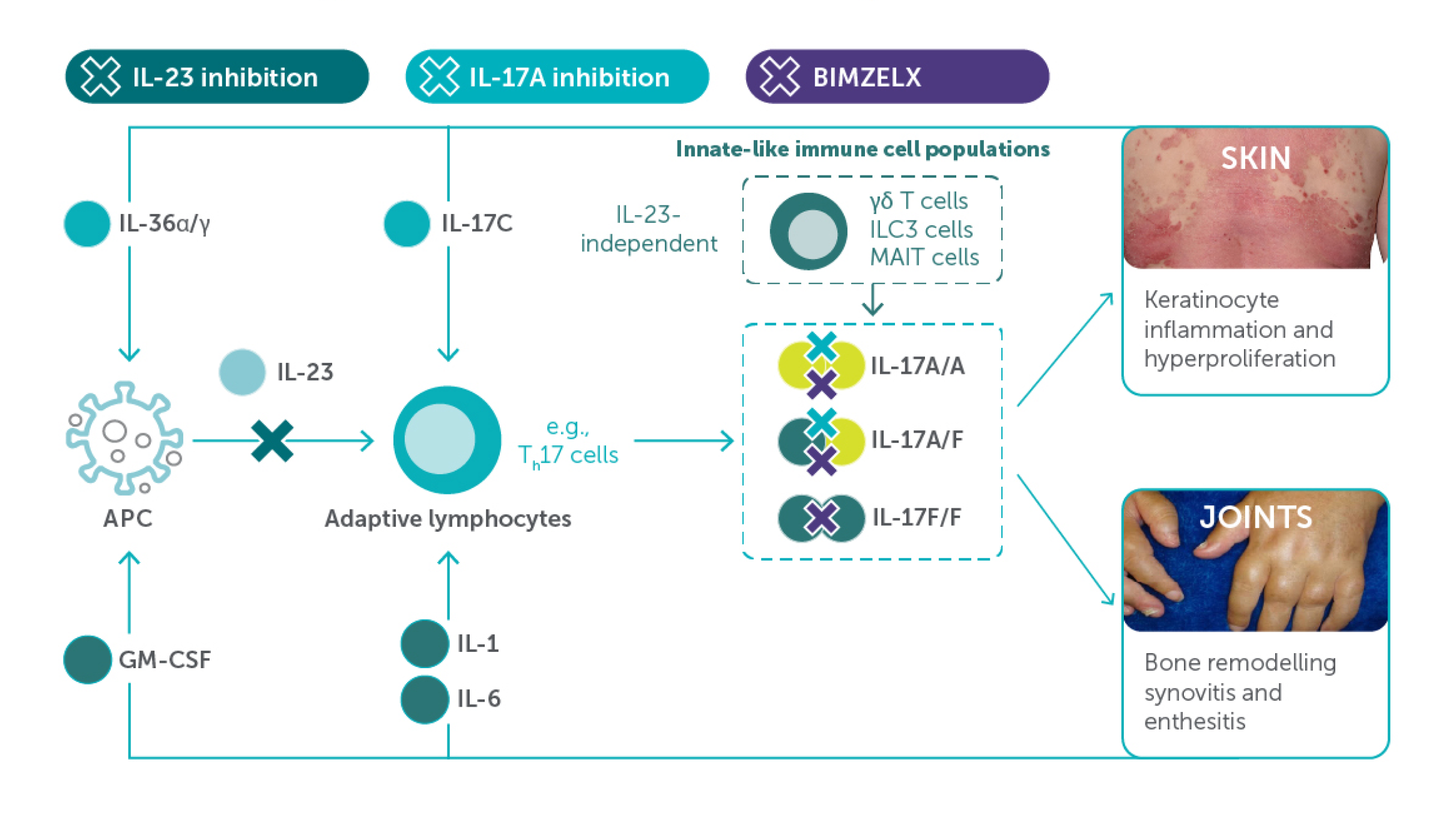
Figure adapted from references 3–11.
APC, antigen-presenting cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; ILC3, type 3 innate lymphoid cells; MoA, mechanism of action; MAIT, mucosal-associated invariant T; Th17, T helper 17.
References: 1. BIMZELX SmPC. 2. Mease P, et al. Rheumatol. 2024;63:1779–89. 3. Cole S, et al. Front Immunol. 2020;11:585134. 4. Glatt S, et al. Ann Rheum Dis. 2018;77:523–32. 5. Oliver R, et al. Br J Dermatol. 2022;186:652–63. 6. Marinoni B, et al. Auto Immun Highlights. 2014;5:9–19. 7. Fuentelsaz-Romero S, et al. Front Immunol. 2021;11:613975. 8. Benham H, et al. Arthritis Res Ther. 2013;15:R136. 9. Sakkas LI, et al. Front Pharmacol. 2019;10:872. 10. Rosine N, et al. Front Immunol. 2021;11:553742. 11. Chimenti MS, et al. Front Immunol. 2018;30:9:236.
Skin Efficacy
BIMZELX demonstrated improved skin efficacy vs three other biologic therapies1–4
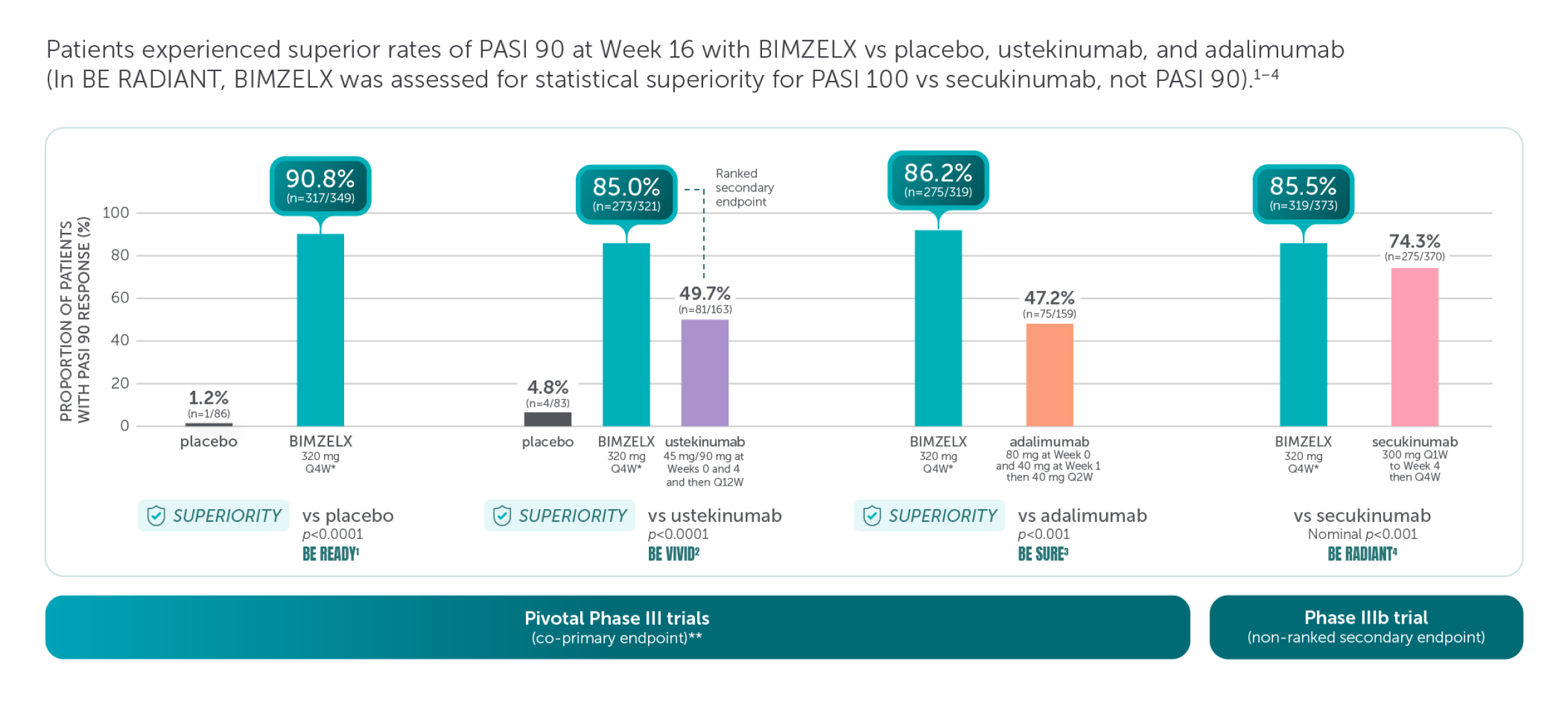
These are four separate studies and should not be directly compared. Adapted from references 1–4.
Missing data were imputed with non-responder imputation; p values for the comparison of treatment groups were based on CMH test from the general association.1–4
*The recommended dose for adult patients with plaque PsO is 320 mg (given as two subcutaneous injections of 160 mg or one subcutaneous injection of 320 mg) at Week 0, 4, 8, 12, 16 and every 8 weeks thereafter.5
**The co-primary efficacy endpoints for the pivotal phase III studies BE READY, BE VIVID, and BE SURE were PASI 90 and IGA score of clear or almost clear (IGA 0/1) at Week 16.1–4 †The primary endpoint of BE RADIANT was PASI 100 response at Week 16 with BIMZELX vs secukinumab.4
aRD, adjudicated risk difference; CI, confidence interval; CMH, Cochran-Mantel-Haenszel; PASI 90, ≥90% improvement from baseline in Psoriasis Area and Severity Index; Q1W, once every week; Q2W, every 2 weeks; Q4W, every 4 weeks; Q12W, every 12 weeks.
References: 1. Gordon KB, et al. Lancet. 2021;397:475–486. 2. Reich K, et al. Lancet. 2021;397:487–498. 3. Warren RB, et al. N Engl J Med. 2021;385:130–141. 4. Reich K, et al. N Engl J Med. 2021;385:142–152. 5. BIMZELX SmPC.
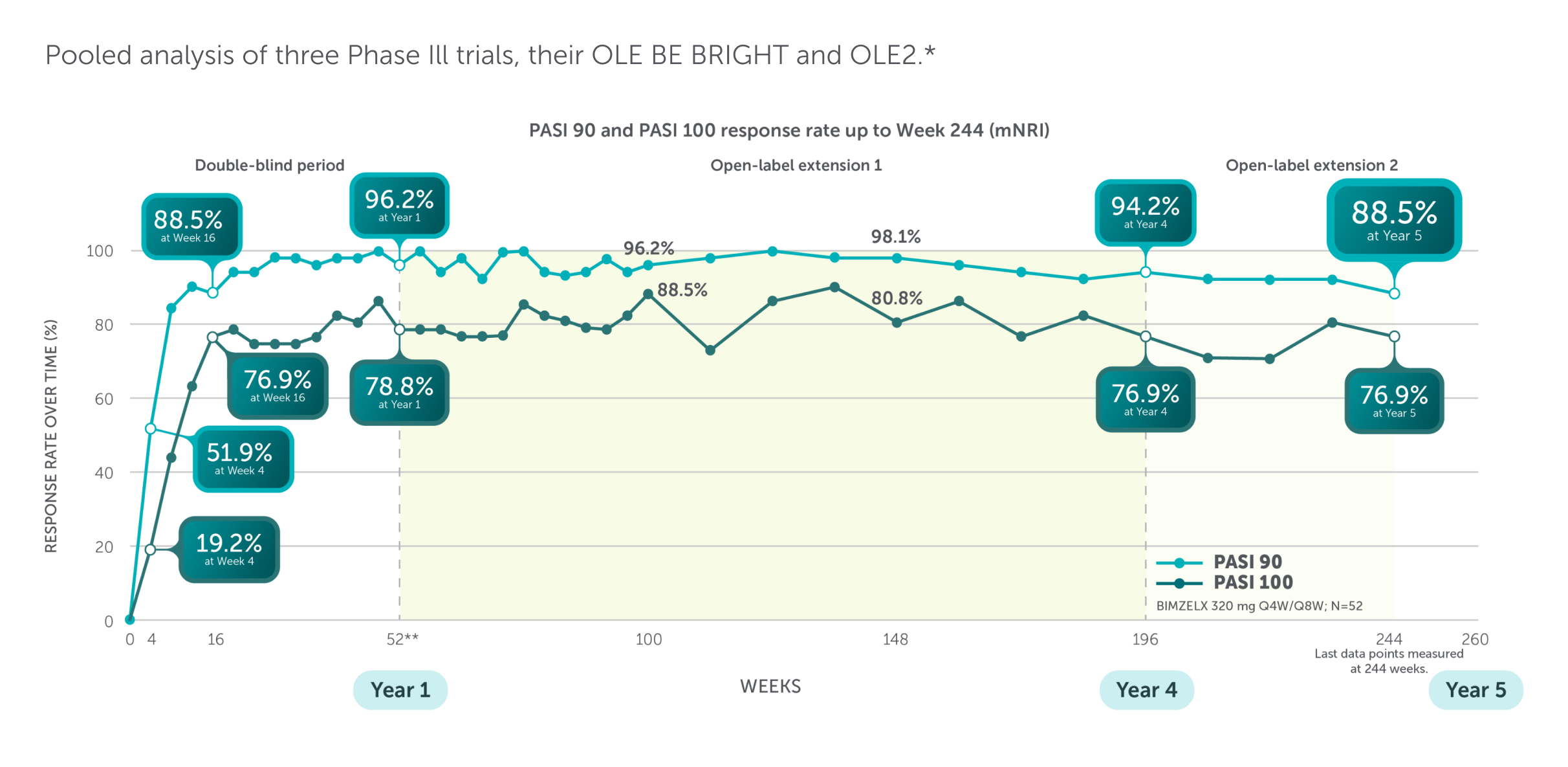
Bimzelx demonstrated lasting skin efficacy that was rapid in onset
To understand more about our Phase III study designs, please click here
*Patients who completed BE SURE, BE VIVID, or BE READY were eligible to enrol in BE BRIGHT, an OLE study designed to assess long-term safety, tolerability, and efficacy of BIMZELX. US/Canadian patients who completed the BE BRIGHT OLE (4 years’ total treatment), could enter a second 48-week OLE (OLE2). The mean weight of US/Canadian patients in OLE2 was numerically higher than the global BE BRIGHT population (mean weight was 88.5 ± 20.8 kg [BIMZELX Q4W/Q8W]). **BE VIVID lasted 52 weeks and BE SURE and BE READY lasted 56 weeks; to pool data across studies, Week 56 data were not included. In this graph, the period after Week 52 (or Week 48/52) corresponds to the BE BRIGHT OLE.
(m)NRI, (modified) non-responder imputation: OLE, open-label extension; PASI 90/100, ≥90/100% improvement from baseline in Psoriasis Area and Severity Index; Q4W, every 4 weeks; Q8W, every 8 weeks.
Reference: Blauvelt. 2025. AAD Presentation 62275.
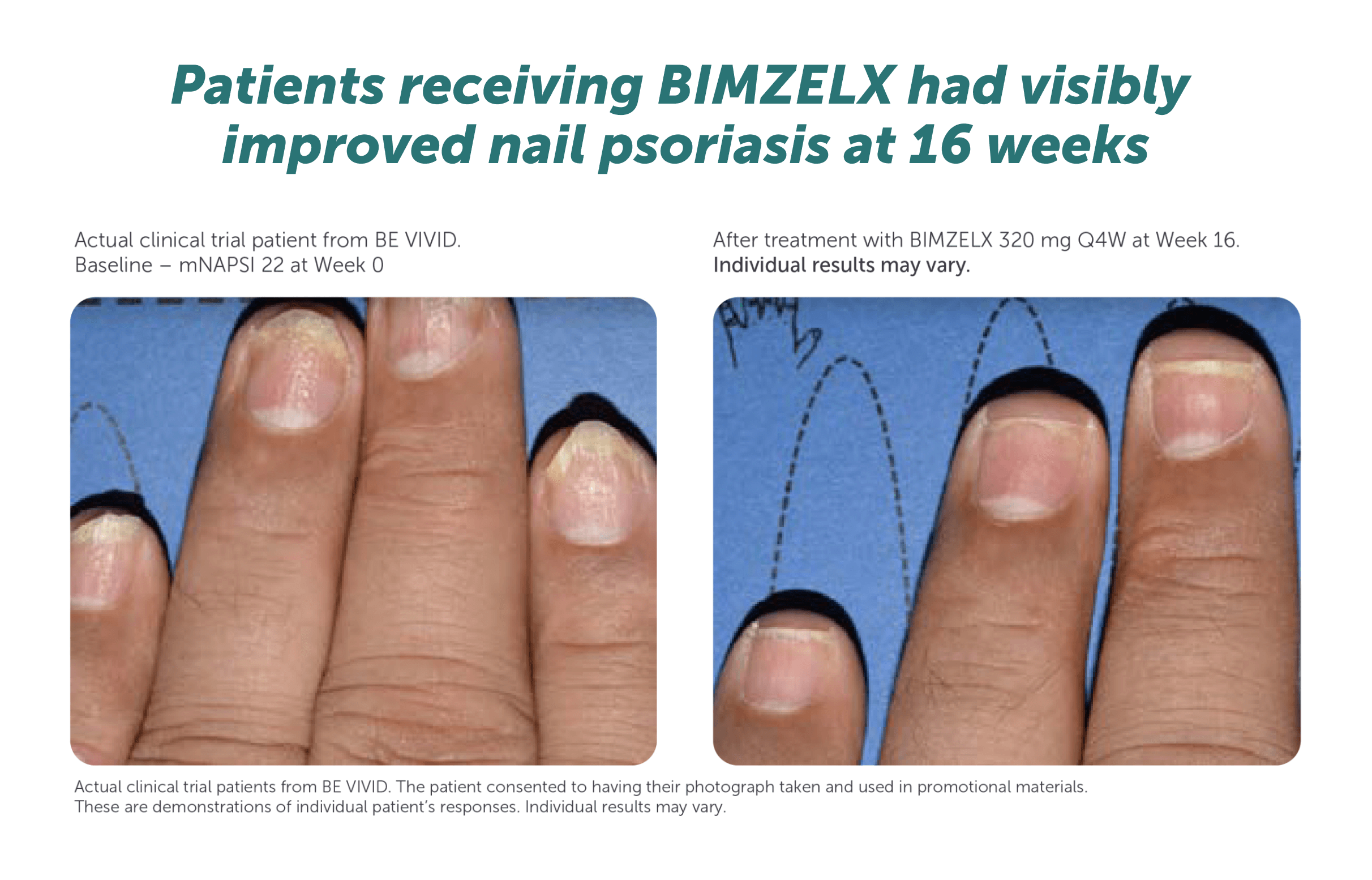
Patients receiving BIMZELX had visibly improved skin clearance at 16 weeks1–4
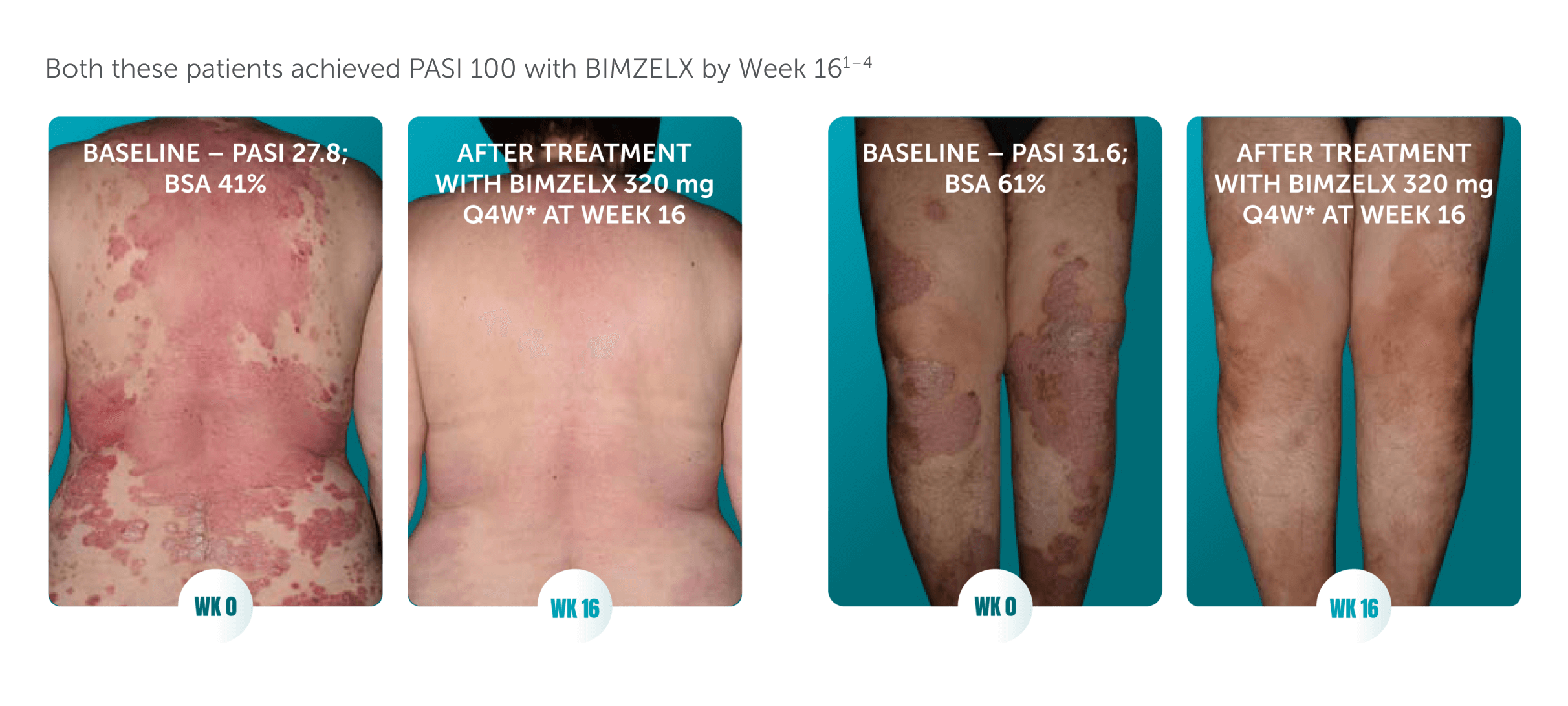
Actual clinical trial patients from BE RADIANT (left)1,2 and BE VIVID (right).3,4 The patients consented to having their photograph taken and used in promotional materials. These are demonstrations of individual patient’s responses. Individual results may vary.
BSA, body surface area; PASI 100, 100% improvement from baseline in Psoriasis Area and Severity Index; Q4W, every 4 weeks; WK, week.
References: 1. Reich K, et al. N Engl J Med. 2021;385:142–152. 2. UCB Data on file. 2020 BE RADIANT – patient photos. 3. Reich K, et al. Lancet. 2021;397:487–498. 4. UCB Data on file. 2020 BE VIVID – patient photos. 5. BIMZELX SmPC.
High Impact Areas
BIMZELX demonstrated high rates of complete clearance for high-impact sites up to 4 years1
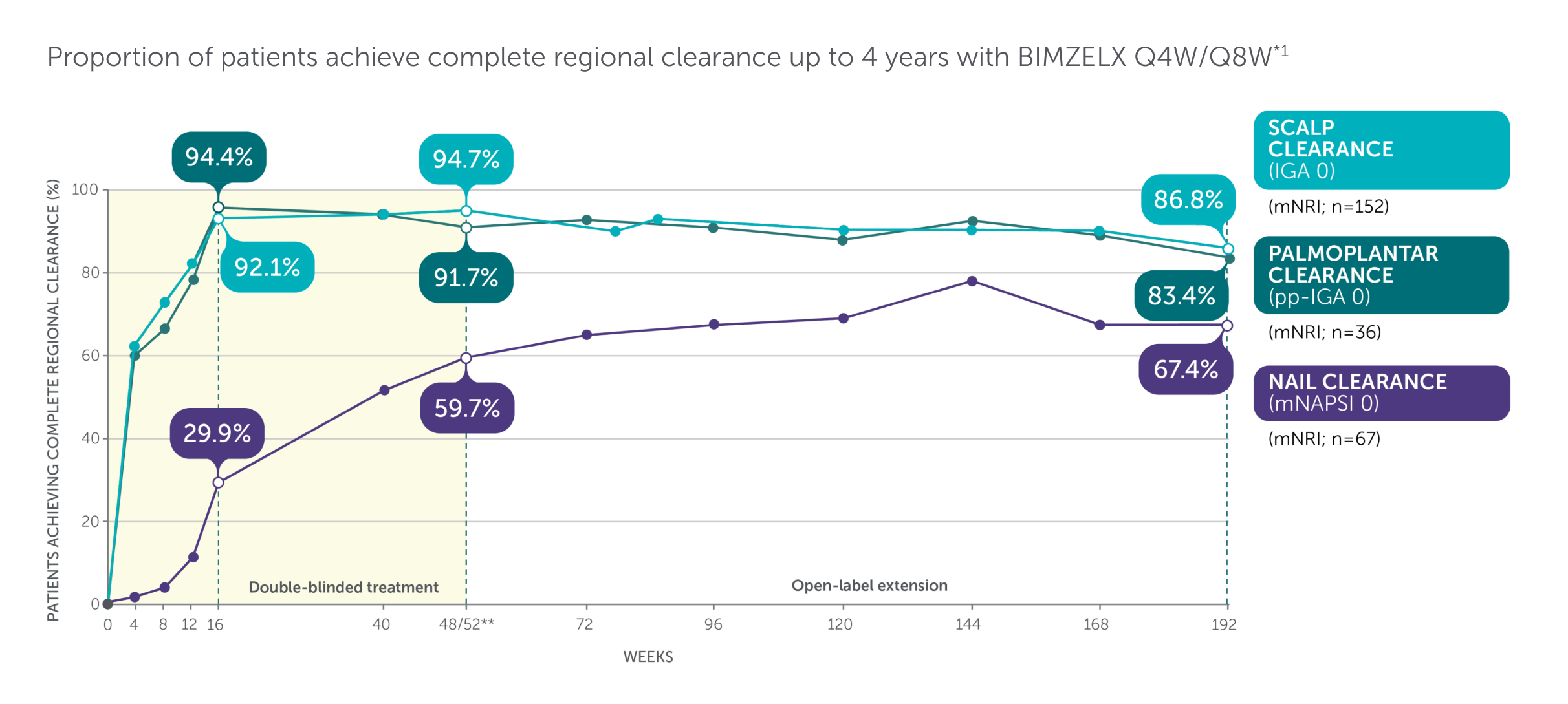
Adapted from Merola 2024.1
Includes patients with IGA ≥3. mNAPSI >10, or pp-IGA ≥3 at baseline. *Data are reported using modified non-responder imputation (mNRI): patients who discontinued due to lack of efficacy or treatment-related adverse events were considered non-responders; multiple imputation was used for other missing data. mNRI for scalp clearance, n=152. mNRI for nail clearance, n=67. mNRI for palmoplantar clearance, n=36. **Week 48/52 data are from Week 48 of BE SURE and BE READY, and Week 52 of BE VIVID, due to differences in assessment schedules.1 Post-hoc analysis of data pooled from BE VIVID (52 weeks), BE READY and BE SURE (56 weeks), and 3 years of their OLE, BE BRIGHT.1 BIMZELX Q4W/Q8W patients received BIMZELX 320 mg Q4W to Week 16, then BIMZELX Q8W throughout the maintenance period and on OLE entry.1 Scalp IGA was a listed original secondary endpoint for BE READY and BE VIVID.2,3 Change in mNAPSI score and pp-IGA response (in patients with nail and palmoplantar psoriasis at baseline) were exploratory endpoints in BE READY and BE VIVID.4,5
IGA, Investigator’s Global Assessment; mNAPSI, modified Nail Psoriasis Severity Index; mNRI, modified non-responder imputation; OLE, open-label extension; pp, palmoplantar psoriasis; Q4W, every 4 weeks; Q8W, every 8 weeks.
References: 1. Merola JF. EADV 2024; P3320. 2. Gordon KB, et al. Lancet. 2021;397(10273):475–486. 3. Reich K, et al. Lancet. 2021;397:487–498. 4. Gordon KB, et al. Lancet. 2021;397(10273):475–486. Supp App. 5. Reich K, et al. Lancet. 2021;397:487–498. Supp App.
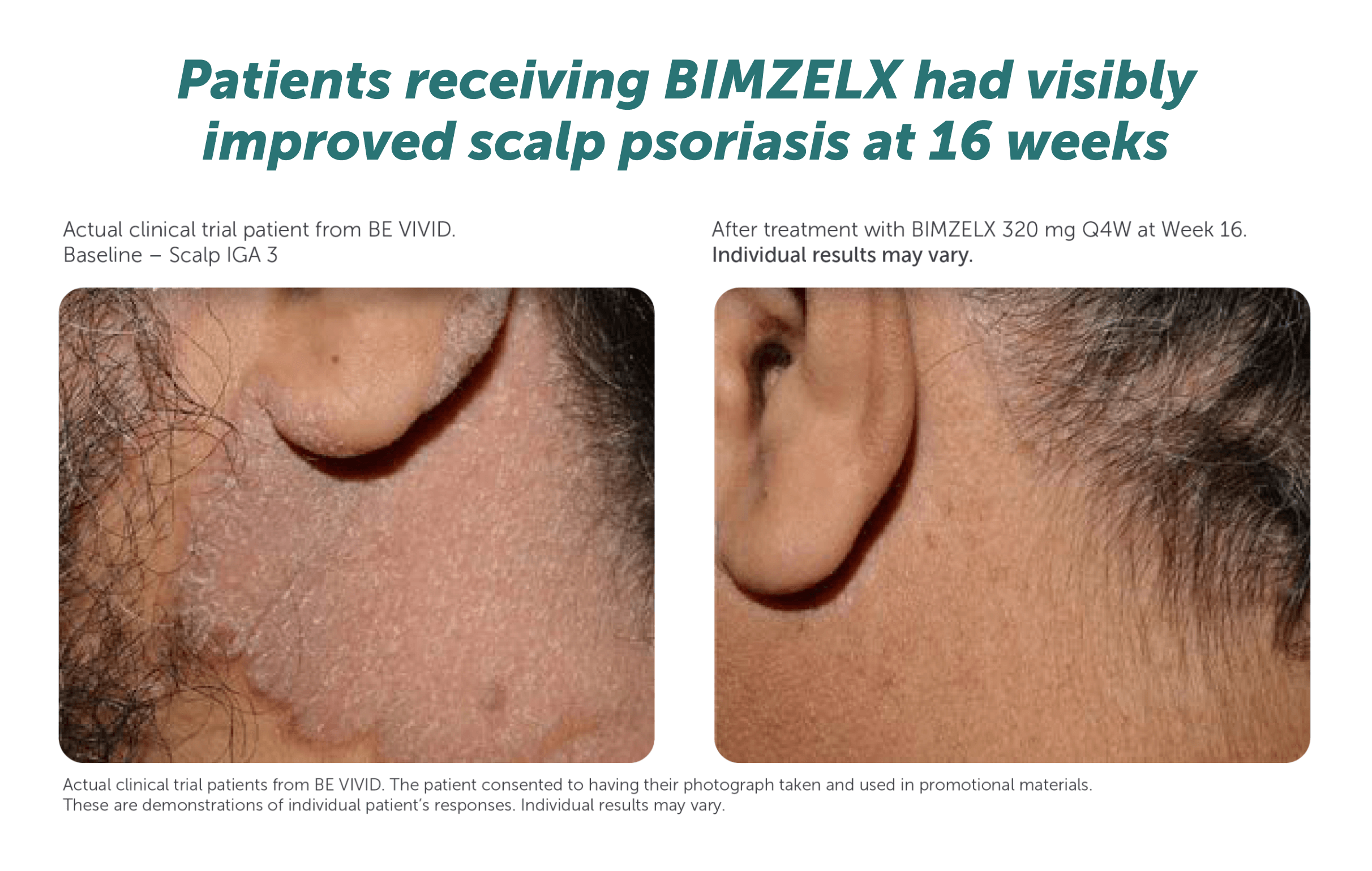
PSA Efficacy
BIMZELX demonstrated clinically meaningful joint improvement for patients with PsA
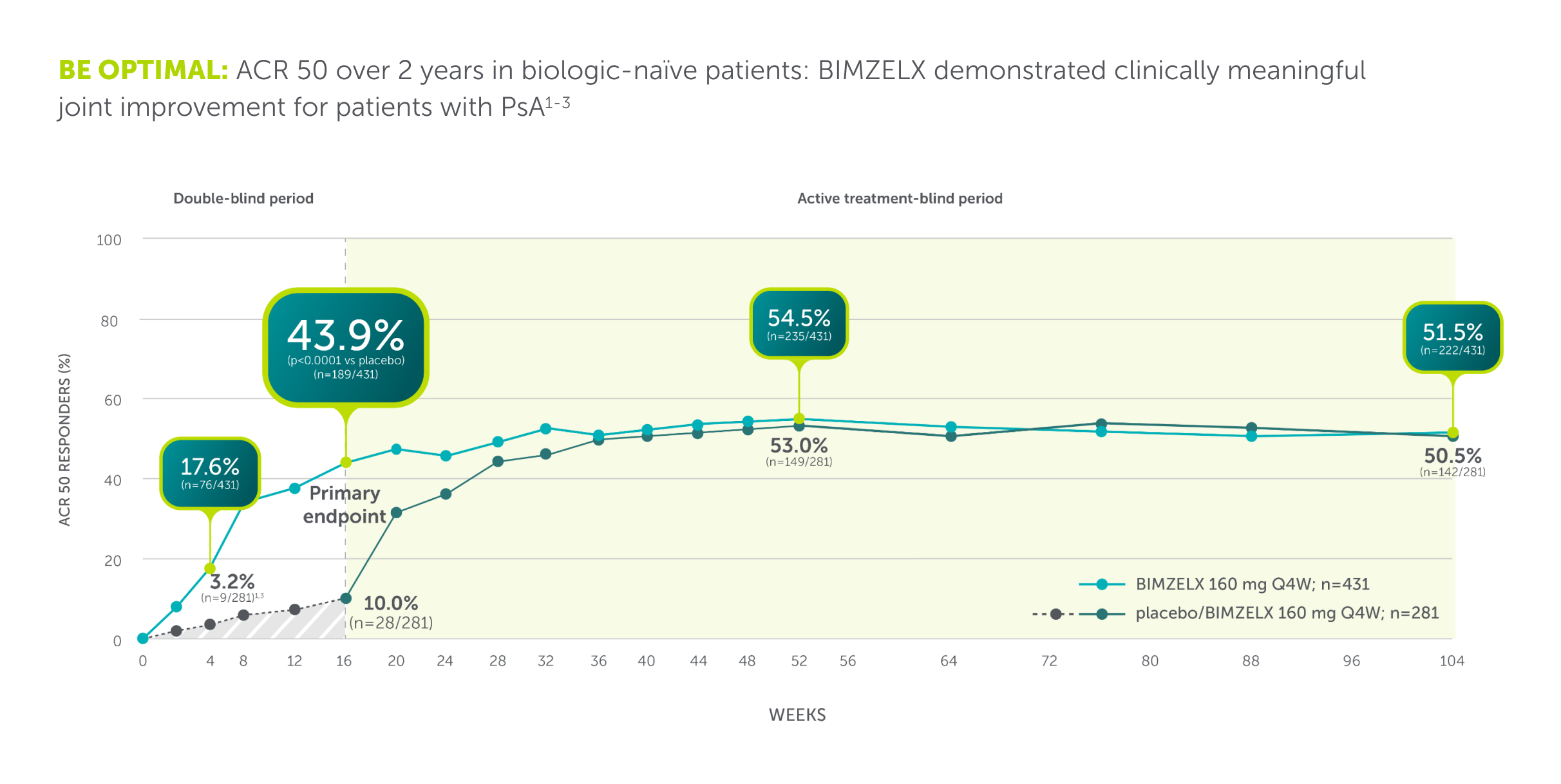
BE OPTIMAL: Phase III study (N=852) assessing efficacy and safety of BIMZELX in patients with active PsA who were naïve to bDMARDs. The primary endpoint was ACR 50 at Week 16. Patients were randomised 3:2:1 to BIMZELX 160 mg Q4W (n=431), placebo Q2W (n=281), or adalimumab 40 mg Q2W (active reference; n=140). After Week 16, all patients were aware that they were receiving active treatment, which may have affected the results. Missing data were imputed with NRI and p values were generated using logistic regression.2
ACR 50, ≥50% response in the American College of Rheumatology criteria; BSA, body surface area; DMARD, biologic disease-modifying antirheumatic drug; NRI, non-responder imputation; PsA, psoriatic arthritis; Q2W, every 2 weeks; Q4W, every 4 weeks.
References: 1. Mease PJ, et al. Rheumatol Ther. 2024;11(5):1363–1382. 2. Ritchlin CT, et al. Ann Rheum Dis. 2023; 82(11):1404–1414. 3. McInnes IB, et al. Lancet. 2023;401:25–37.
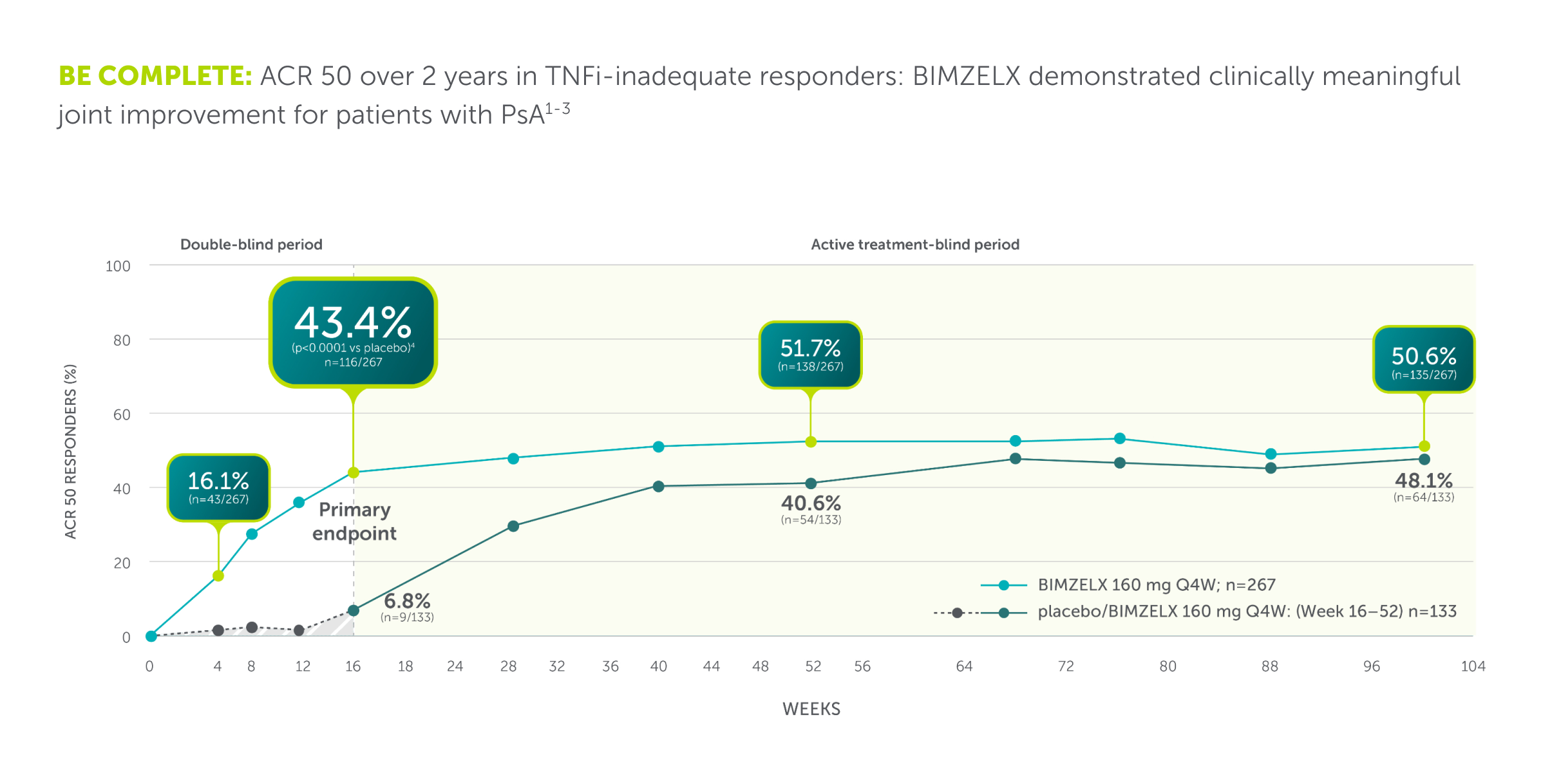
ACR 50, ≥50% response in the American College of Rheumatology criteria; NRI, nonresponder imputation; PsA, psoriatic arthritis; Q4W, every 4 weeks; TNFi-IR, tumour necrosis factor-α inhibitor - inadequate responder.
References: 1. Mease PJ, et al. Rheumatol Ther. 2024;11(5):1363–1382. 2. Coates LC, et al. RMD Open. 2024;10(1):e003855. 3. Merola JF, et al. Lancet. 2023;401:38–48
Safety Profile
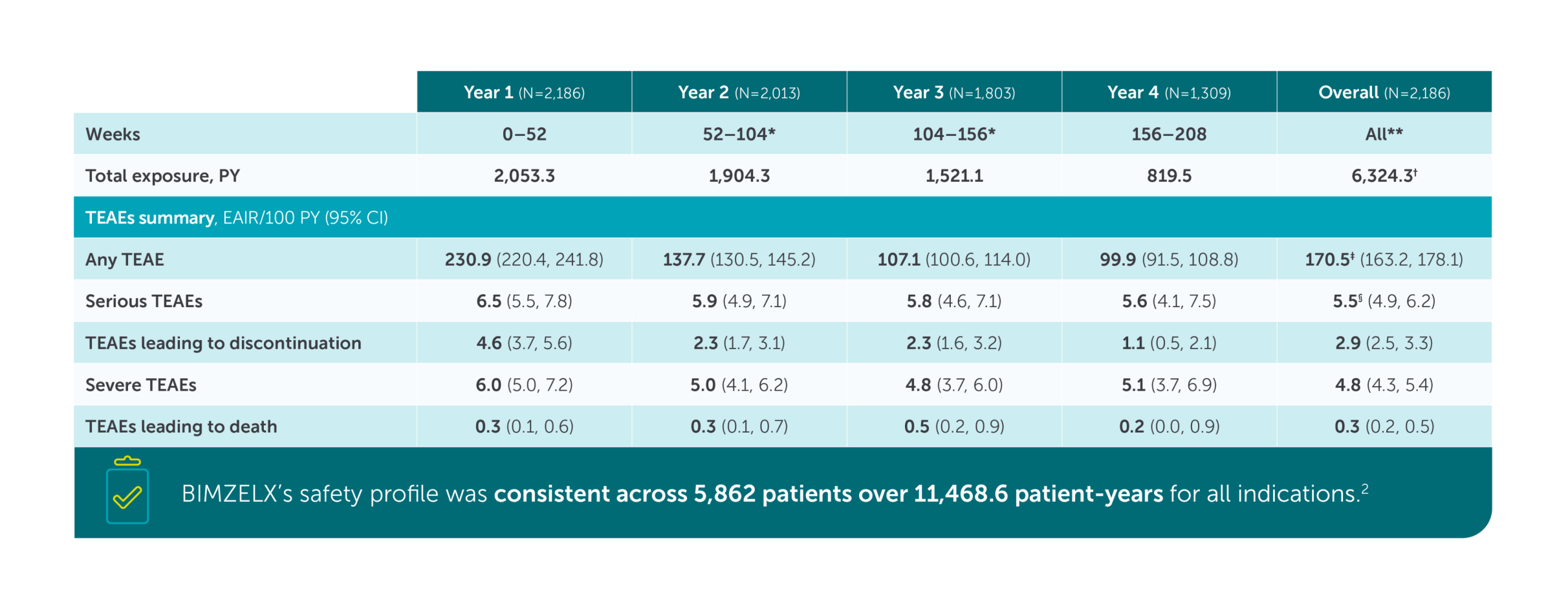
BIMZELX demonstrated a consistent safety profile over 4 years among patients with PsO1
In a pooled analysis of three Phase III trials (BE VIVID, BE READY and BE SURE) and their OLE BE BRIGHT, and the phase Illb trial and OLE study BE RADIANT, EAIR per 100 PY of serious and severe TEAEs were low with BIMZELX treatment over longer-term treatment exposure1
Contraindictions
Bimekizumab is contraindicated in patients with clinically important active infections (e.g., active tuberculosis) or known hypersensitivity to the active substance or any excipients listed in section 6.1 of the SmPC.
Warnings and precautions
Bimekizumab may increase the risk of infections, including upper respiratory tract infections and oral candidiasis. Use with caution in patients with chronic or recurrent infections, and do not start treatment in those with active, clinically significant infections until resolved. Patients should seek medical advice if infection symptoms occur. If an infection develops, monitor closely and discontinue treatment if it becomes serious or unresponsive to standard therapy.
Additional Warnings and Precautions
Bimekizumab has been associated with new or worsening cases of inflammatory bowel disease (IBD) and is not recommended for patients with IBD. If signs or symptoms of IBD occur or existing IBD worsens, discontinue treatment and initiate appropriate medical care.
- Traceability: Record product name and batch number to ensure traceability.
- Tuberculosis (TB): Screen for TB before starting. Do not use in active TB. Monitor for TB symptoms during treatment; consider prophylaxis if past TB treatment is uncertain.
- Vaccinations: Complete all age-appropriate immunisations before starting. Avoid live vaccines during treatment; inactivated vaccines are permitted.
- Excipients: Contains polysorbate 80 (may cause allergies) and is essentially sodium-free.
You are advised to refer to the SmPC for full details and further information.
*All patients were switched to BIMZELX 320 mg Q8W at the next scheduled clinic visit on or after the Week 64/Week 104 visit (BE RADIANT/BE BRIGHT, respectively) following protocol amendment. **Entire pooled study period. †Total BIMZELX exposure over 4 years is greater than the sum of BIMZELX exposure in individual years, as data beyond Week 208 are not included due to the use of a cutoff date. ‡The EAIR of TEAEs over 4 years was numerically lower in patients receiving BIMZELX Q8W vs Q4W (115.4/100 PY vs 224.4/100 PY). §The rate of serious TEAEs over 4 years is lower than the rate in any individual year due to time not accounted for in the individual year summaries.
Cl, confidence interval; EAIR, exposure-adjusted incidence rate; PY, patient-year; Q4W, every 4 weeks; Q8W, every 8 weeks; TEAE, treatment-emergent adverse event.
References: 1. Gordon. 2024. AAD. Presentation 52671. 2. BIMZELX SmPC.
Prescribing & Dosing
Six to seven maintenance doses a year for your patients with moderate-to-severe plaque PsO1

The recommended BIMZELX dose for adult patients with plaque PsO is 320 mg (given as two subcutaneous injections of 160 mg or one subcutaneous injection of 320 mg) at Week 0, 4, 8, 12, 16 and every 8 weeks thereafter. This equates to 9 doses in Year 1 then 6 or 7 doses per year thereafter.
Consideration should be given to discontinuing treatment in patients who have shown no improvement by 16 weeks of treatment. Overweight patients: For some patients with a body weight ≥120 kg who do not achieve complete skin clearance at Week 16, BIMZELX 320 mg Q4W after Week 16 may further improve treatment response.1
References: 1. BIMZELX SmPC.
Study details
BIMZELX was assessed across four Phase III/IIIb trials in adult patients with moderate-to-severe PsO1–4
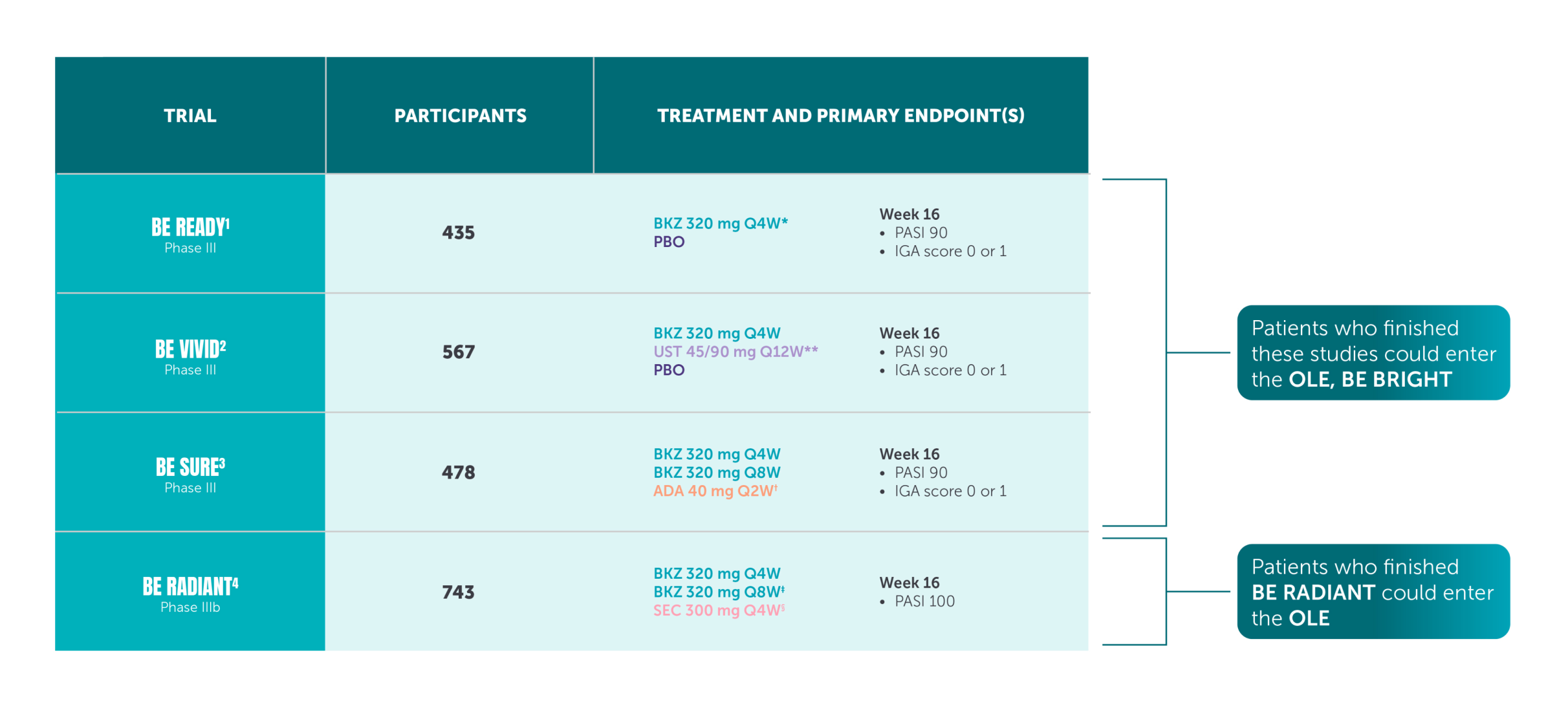
Open-label extension data are also available for all trials. The bimekizumab clinical trials included some off-label treatment arms. Please refer to the SmPC for the licenced indications. Bimekizumab is indicated for the treatment of moderate-to-severe plaque PsO in adults who are candidates for systemic therapy.
*After Week 16, patients receiving BKZ achieving PASI 90 were re-allocated (1:1:1) to receiving BKZ 320 mg Q4W, Q48, or placebo for Weeks 16–56.1
**Patients received 45/90 mg at Weeks 0 and 4, then Q12W. UST dosing was based on weight plan: patients ≤100 kg at baseline received one UST 45 mg injection; patients >100 kg at baseline received two.2
†ADA was dosed 80 mg at Week 0 and 40 mg at Week 1, then Q2W until Week 24 where the patient was switched to BKZ 320 mg Q4W.3
‡The Q8W maintenance dosing regimen was added via protocol amendment, with the first re-randomisation occurring ~7 months after the trial began, after 82 patients had completed Week 16.
§SEC was administered weekly until Week 4 and then monthly.
References: 1. Gordon KB, et al. Lancet. 2021;397:475–486. 2. Reich K, et al. Lancet. 2021;397:487–498. 3. Warren RB, et al. N Engl J Med. 2021;385:130–141. 4. Reich K, et al. N Engl J Med. 2021;385:142–152. 5. BIMZELX SmPC.
Prescribing Information for Healthcare Professionals in the United Kingdom, click here
▼ This medicine is subject to additional monitoring. This will allow quick identification of new safety information. Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk for United Kingdom and www.hpra.ie/homepage/about-us/report-an-issue for Republic of Ireland. Adverse events should also be reported to UCB Pharma Ltd at UCBCares.UK@UCB.com or 0800 2793177 for the UK and UCB (Pharma) Ireland Ltd at UCBCares.IE@UCB.com or 1800 930075 for Republic of Ireland
Prescribing Information for Healthcare Professionals in the Republic of Ireland
Prescribing Information for HCP's in Republic of Ireland
(Please consult the Summary of Product Characteristics (SmPC) before prescribing)

