and that’s okay.”
Living with gMG


of myasthenia gravis (MG) patients may have
uncontrolled and debilitating symptoms,
despite treatment with conventional therapies1–3

of myasthenia gravis (MG) patients may have uncontrolled and debilitating symptoms, despite treatment with conventional therapies1–3
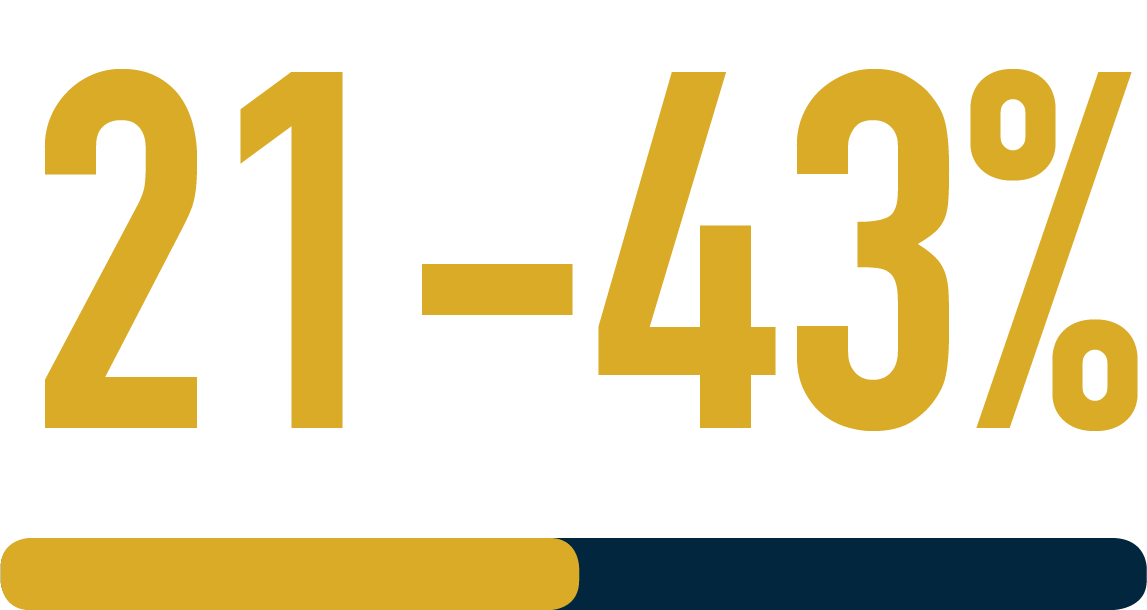
of refractory MG patients experienced at least one emergency hospital visit, were hospitalised overnight at least once or required a feeding tube over the course of 6 months3*
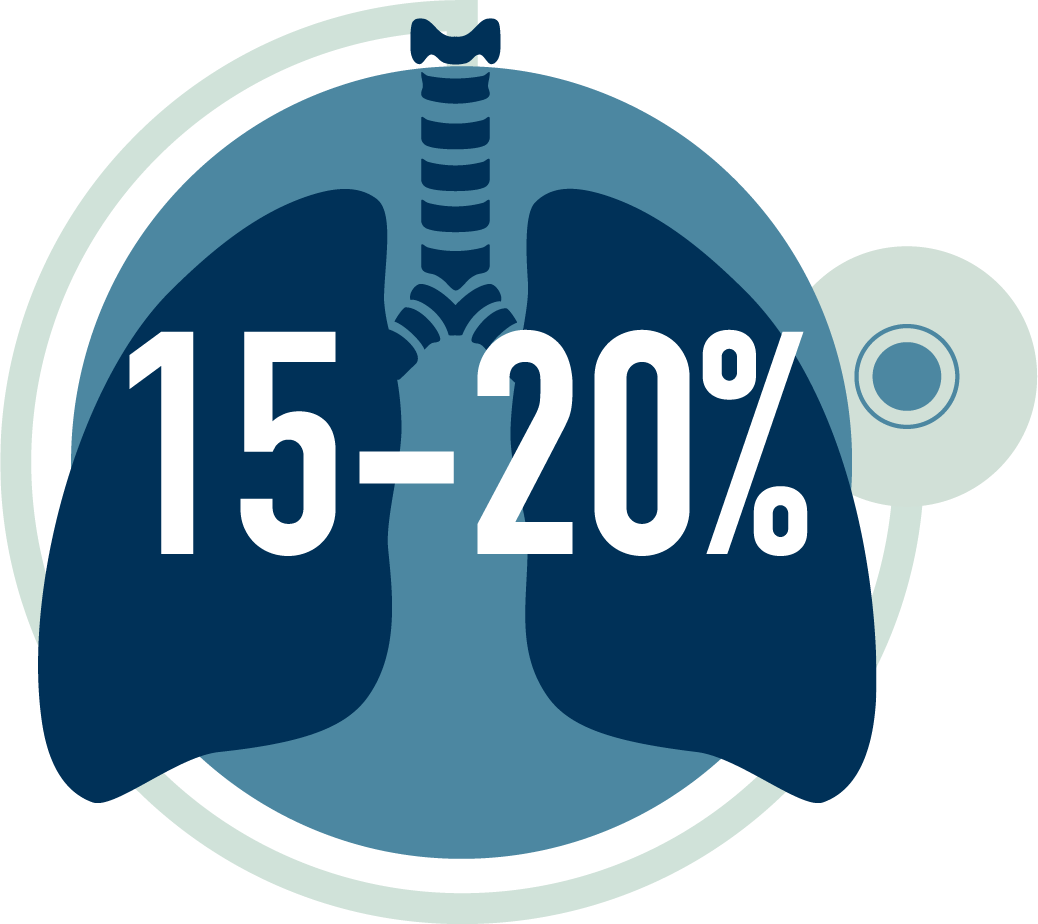
of MG patients experience a potentially life-threatening myasthenic crisis during their lifetime that requires mechanical ventilation3,7†

of patients do not reach complete
stable remission8,9‡
UCB is a global biopharma company, inspired by the needs of people living with severe diseases
Our ambition is to advance the standard of care in gMG
At UCB, we create life-changing therapies for people with neurological conditions and have a decades-long record of innovation in neurology, including breakthrough product launches and philanthropic endeavours.10
We seek to elevate the patient care experience by offering well-designed clinical trials, innovative treatments and non-drug solutions that aim to provide personalised support for patients, when they need it most.10
UCB recently worked with patients along with clinical experts and regulatory agencies to develop the Myasthenia Gravis Symptoms Patient-Reported Outcome (MGSPRO), a novel PRO measure of real patients’ lived experiences with MG.11,12
Find out more about gMG
References