gravis (gMG) affects many aspects
of patients’ lives

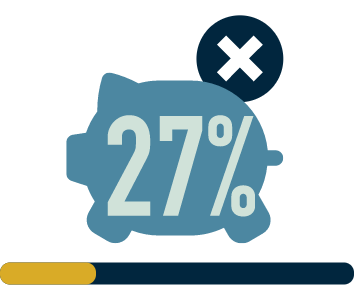
96% (n=27/28)
experience impacts
on their social life2
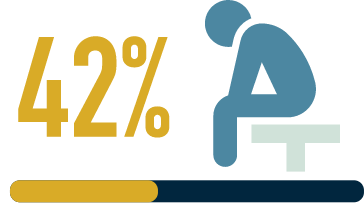
(n=36) have experienced
at least one major
depressive episode
within their lifetime3
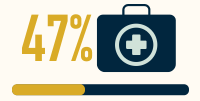
unable to work due to the effects of their disease (n=165)5
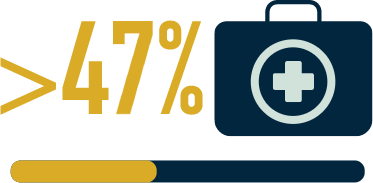
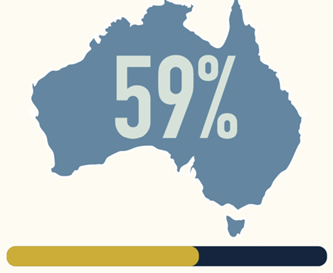
(n=136/330) face ≥9 weeks
of sickness absence
from work in the first
2 years after diagnosis6
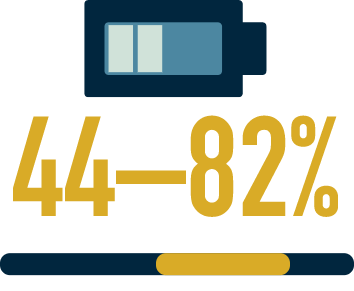
are fatigued7
So may struggle to meet up
with family and friends
So may have a reduced
health-related quality of
life (HRQoL) and increased
burden on caregivers4
So may struggle financially
So may miss out on
work-related opportunities
and progression
So may not be able to plan
for the long term
is a core clinical manifestation
of myasthenia gravis (MG)9 Fatigability is a measurable change in muscle strength over a given time, with symptoms developing or worsening during sustained activity11
In neuromuscular disorders, muscle fatigability is the direct result of the dysfunction of the muscle or the neuromuscular junction (NMJ)8
Understanding of fatigue is limited, but the pathophysiology of fatigue in neuromuscular disorders is believed to have a role in protecting muscles from further damage by downregulating physical activities8,12
- Fatigue may keep individuals from performing daily activities and lower their QoL, which in turn may decrease motivation and further increase fatigue11
- Fatigue can be misinterpreted for laziness in the workplace, which can contribute to the social and professional disadvantages many patients with gMG experience6
Fatigue in MG is associated with higher disease severity,
higher rates of depression, and lower
quality of life8,10
Clinical assessments often exclude fatigue, and studies often use different questionnaires to measure it, but there is a need for consensus and further studies with fatigue as a primary endpoint8,11
The recently developed Myasthenia Gravis Symptoms Patient-Reported Outcome (MGSPRO) scales contain a detailed assessments of muscle weakness (ocular, bulbar and respiratory), muscle weakness fatigability and an assessment of physical fatigue, an aspect not included in other PRO instruments15,16
- Unlike other PRO instruments, the MGSPRO contains a more granular assessment of muscle weakness and muscle weakness fatigability symptoms, and was designed with patient input at every stage
Recent studies used the Quality of Life in Neurological Disorders Fatigue (Neuro-QoL Fatigue) subscale to assess fatigue in gMG, and evidence supports the Short Form (SF) as a valid and reliable measure17,18
patient-reported and composite measures of gMG disease severity, including fatigue
| Scale | Measures | For people with gMG, improvement could mean: |
|---|---|---|
| Myasthenia Gravis Activities of Daily Living (MG-ADL)19 |
Ocular, bulbar, respiratory, motor/limb impairment19 |
Being able to chat with family and friends without the fear of speech becoming garbled |
| Quantitative Myasthenia Gravis (QMG)20,21 | Ocular, bulbar, respiratory, muscle strength and fatigability20 |
Better hand grip and leg strength |
| Myasthenia Gravis Composite (MGC)20,21 | Ocular, bulbar, respiratory, limbs, neck | Being able to chew and swallow more confidently |
| Myasthenia Gravis Impairment Index (MGII)21,22 | Ocular, bulbar, limb impairment, neck | Reduced fatigability and impairment throughout the day |
| MGSPRO14 | Ocular, bulbar, respiratory, limbs, neck and fatigability |
Having enough energy to last throughout the day |
| Myasthenia Gravis Quality of Life 15-item Scale – Revised (MG-QoL15r)20,21 |
HRQoL as determined by physical, psychological and social aspects of functioning |
Reduced symptom fluctuations, helping work and social life |
| Neuro-QoL-Fatigue-SF11 | General fatigue across 8 patient-reported outcomes |
Having more energy and feeling less tired during the day |
| European Quality of Life 5 Dimensions 5 Level Version (EQ-5D-5L)23 |
A generic HRQoL tool across 5 dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression |
Better QoL due to improvement in mobility and ability to complete daily activities |
Efficient and effective communication between
healthcare professionals (HCPs) and patients is
critical to optimising the treatment regimen and
driving better outcomes

Long-term planning1
Allowing for more
frequent breaks1

Changing or reducing the amount or type of work they do1
Proactively cancelling plans if necessary1
Adapting the ways in which they conduct activities of daily living, such as eating or personal hygiene1

Patients may be reluctant to report the true impact of their symptoms due to concerns about switching treatment1
People with MG may feel disconnected from their HCP due to limited appointment times, a gap in the perception of disease and treatment burden and differences in treatment goals1
The most suitable treatment may vary for each patient, depending on their individual preferences and circumstances24
Patients with chronic immune system disorders are more likely to choose subcutaneous (SC) administration over intravenous (IV) infusion, but some prefer the IV route24
- The majority of patients prefer SC therapies due to the convenience and independence associated with self-administration at home
- The minority of patients favour IV infusions due to the infrequency of treatment and the feelings of safety due to hospital administration
It is important that the method of treatment administration does not add to the burden of a patient’s disease24

to their healthcare team
*Fatigue with a duration of ≥6 months.8
EQ-5D-5L, European Quality of Life 5 Dimensions 5 Level Version; gMG, generalised myasthenia gravis; HCP, healthcare professional; HRQoL, health-related quality of life; IV, intravenous; MG, myasthenia gravis; MG-ADL, Myasthenia Gravis Activities of Daily Living; MGC, Myasthenia Gravis Composite; MGII, Myasthenia Gravis Impairment Index; MGSPRO, Myasthenia Gravis Symptoms Patient-Reported Outcome; MG-QoL15r, Myasthenia Gravis Quality of Life 15-item Scale – Revised; NMJ, neuromuscular junction; Neuro-QoL-Fatigue-SF, Quality of Life in Neurological Disorders Fatigue short form; PRO, patient-reported outcome; QMG, Quantitative Myasthenia Gravis; QoL, quality of life; SC, subcutaneous.
References